Jöns Jacob Berzelius [jœns ˌjɑːkɔb bæɹˈseːliɵs] (20 August 1779 – 7 August 1848) was a Swedis chemist. He worked out the modern technique of chemical formula notation, and together with John Dalton, Antoine Lavoisier, and Rbert Boyle considered of modern of chemistry.
Jons Jacob Berzelius ‘’invented’’ a system of chemical notation in 1811. The system is based on the ‘’law of definite proportions’’, stating that all samples of a given chemical compound have the same elemental composition.
What are chemical formulas?
-Chemical formulas are short-hand representations of compounds using chemical symbols and oxidation numbers. (Oxidation is defined as the interaction between oxygen molecules and all the different substances they may contact, from metal to living tissue)
How to write chemical formulae?
+Binary compound:
-The simplest chemical formulas are for binary compounds: make up of 2 elements. they have a positive half and a negative half. The positive half is written first, the negative second. - The key point to write chemical formulas is the oxidation number /valency/ the charge. We can see it in the Periodic Table directly. With the transition metals and any atoms that act in unusual ways, will be provided. The metals have a positive oxidation number, the non-metals have a negative oxidation number. Special with element hydro, it is non-metal but it has positive charge 1+.
-
- - The sum of the oxidation numbers in a compound must equal zero
- -The oxidation number of an ion is equal to its charge
-
MExample:
- Subscripts: small numbers to the lower right of a symbol. They represent the number of atoms of that element in the compound
In iron (III) oxide: it has 2 iron atoms, 3 oxygen atoms in the compound.
In iron (II) oxide: it has 1 iron atom, 1 oxygen atom in the compound.
Subscript of 1 are “understood”, never written.
Polyatomic ions ( ions made up of more than one atom)
- Write their formulas just like binary compounds
- List of polyatomic ions:
- The polyatomic ion may be either the positive or the negative half of the formula – or both
- If more than one polyatomic ion is needed in a formula, put parentheses around the ion add the subscript outside the parenthesis. This is the only time parenthesis are used in a chemical formula
Magnesium hydroxide
Calcium sulphate
Does the ate present 3 oxygen?
- There are a lot of ions with the –ate: acetate ion, nitrate ion, chlorate ion, sulphate ion, carbonate ion, phosphate ion…
- The ate does not present 3 oxygen. For example: sulphate ion SO4 2-, phosphate ion PO4 3-
- The –ate have 1 more oxygen than the -ites and their charge does not change as the result. For example: nitrate NO3 – then nitrite is NO2 -; phosphate is PO4 3- then phosphate is PO3 3-
- The –ate with charges less than negative 1 ( that is , ions with charge of -2, -3, etc.) can have an H added to them to form new polyatomic ions. For each H added, the charge is increased by a+1. For example: CO3 2- then added H and become HCO3 -
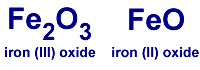



No comments:
Post a Comment